Pre Clinical Assessment - Woodley BioReg Regulatory Affairs, Compliance and Conformance for pharmaceutical, biopharmaceutical, healthcare, API and Medical device manufacturers

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH

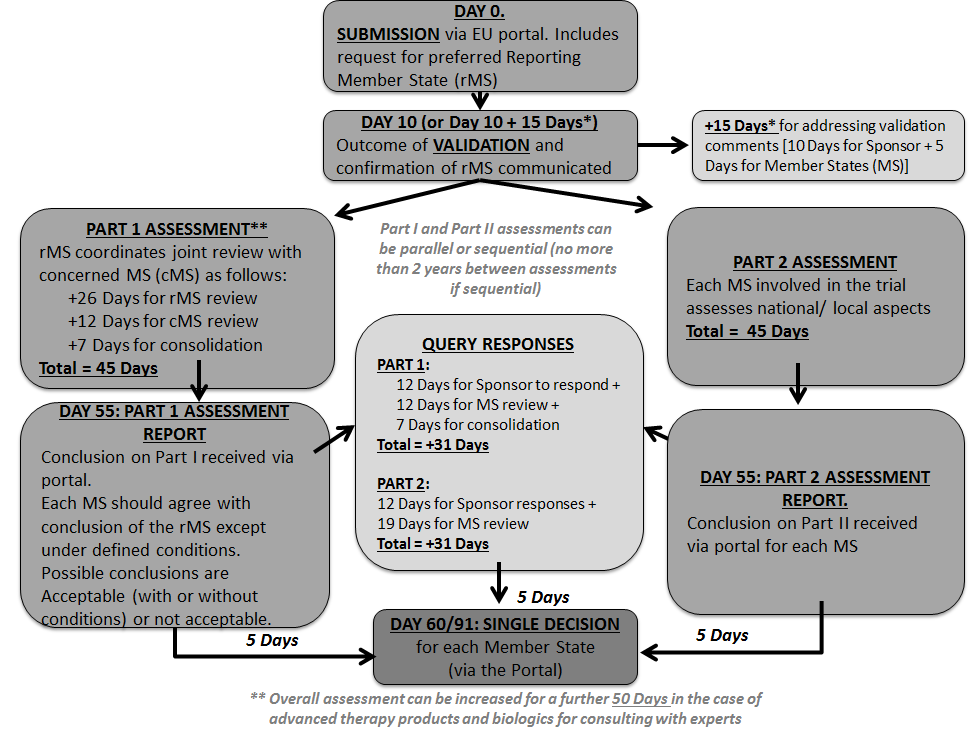
IMPD review process To ensure the implementation of GCP in the MS of EU... | Download Scientific Diagram
What Are the Documents Required for Clinical Trial Applications to Regulatory Authorities in Europe? - Sofpromed

Regulatory Affairs 101: Introduction to Investigational New Drug Applications and Clinical Trial Applications - Chiodin - 2019 - Clinical and Translational Science - Wiley Online Library

Preparation of a Preclinical Dossier to Support an Investigational New Drug (IND) Application and First-In-Human Clinical Trial - ScienceDirect