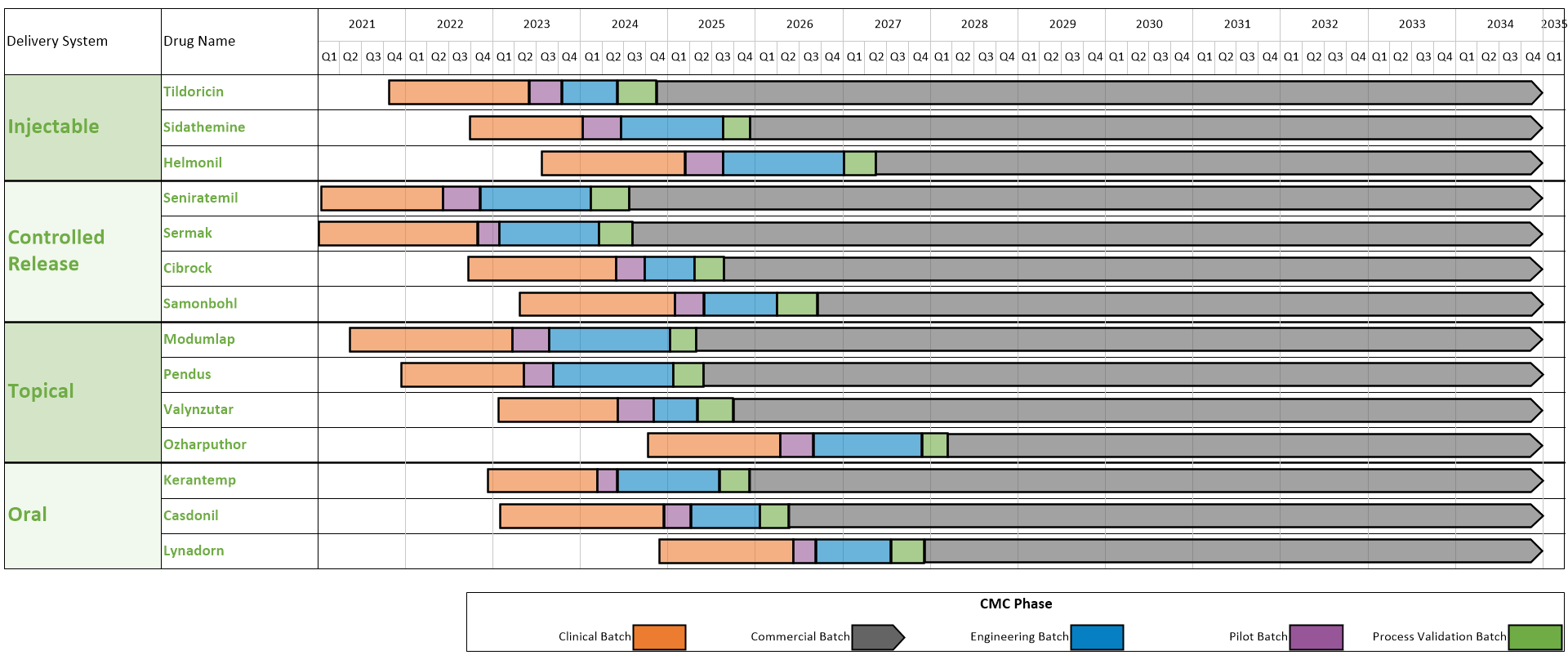
Technology Transfer of CMC Activities for MAb Manufacturing - BioProcess InternationalBioProcess International

Phase-Appropriate CMC Activities Facilitate the Transition from Early Development through Commercialization

SciRhom starts CMC development of its first drug candidate for clinical development | World Pharma Today
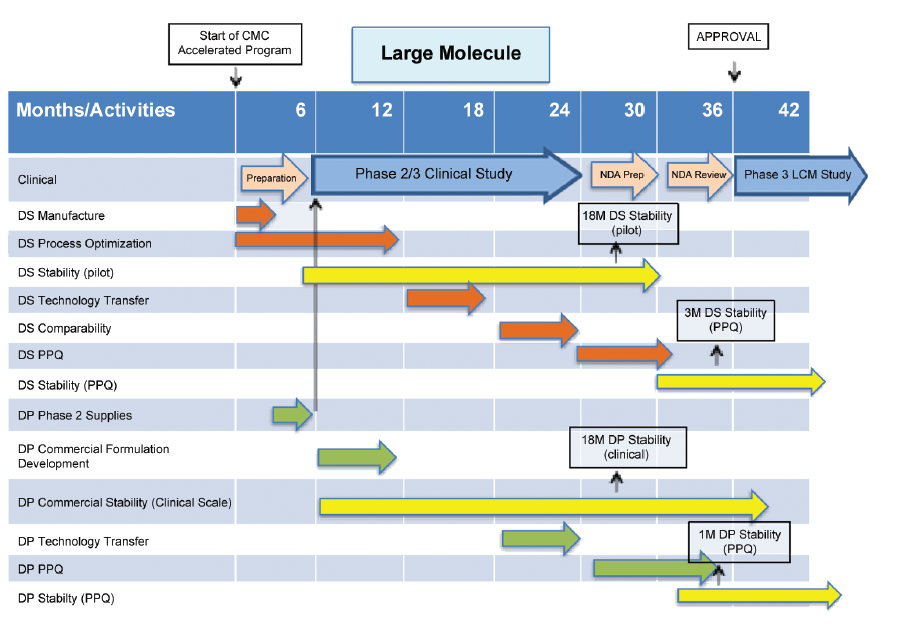
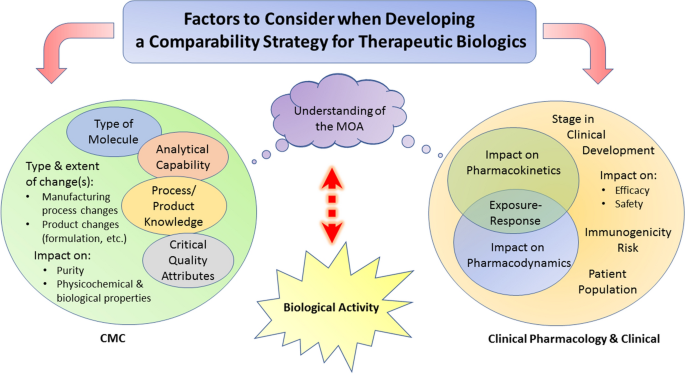
CMC Considerations when a Drug Development Project is Assigned Breakthrough Therapy Status | Pharmaceutical Engineering
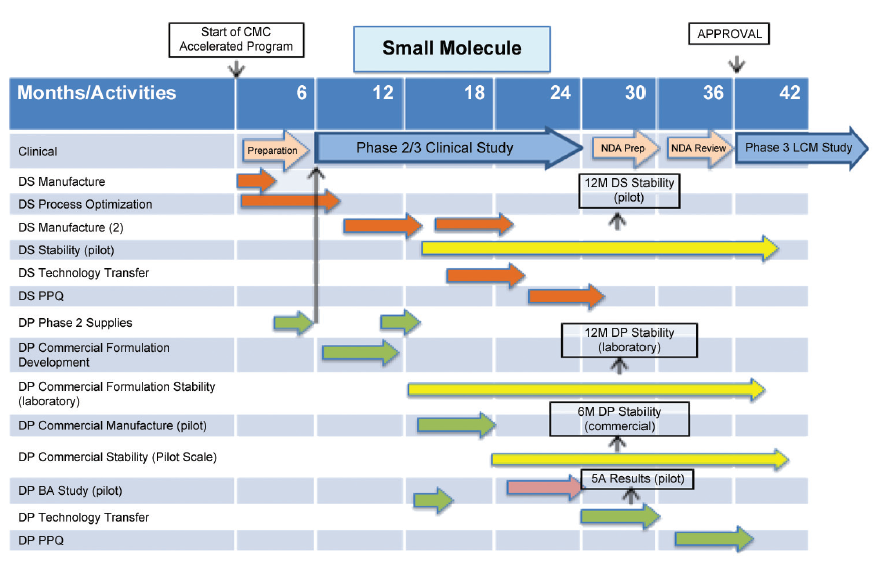
CMC Considerations when a Drug Development Project is Assigned Breakthrough Therapy Status | Pharmaceutical Engineering

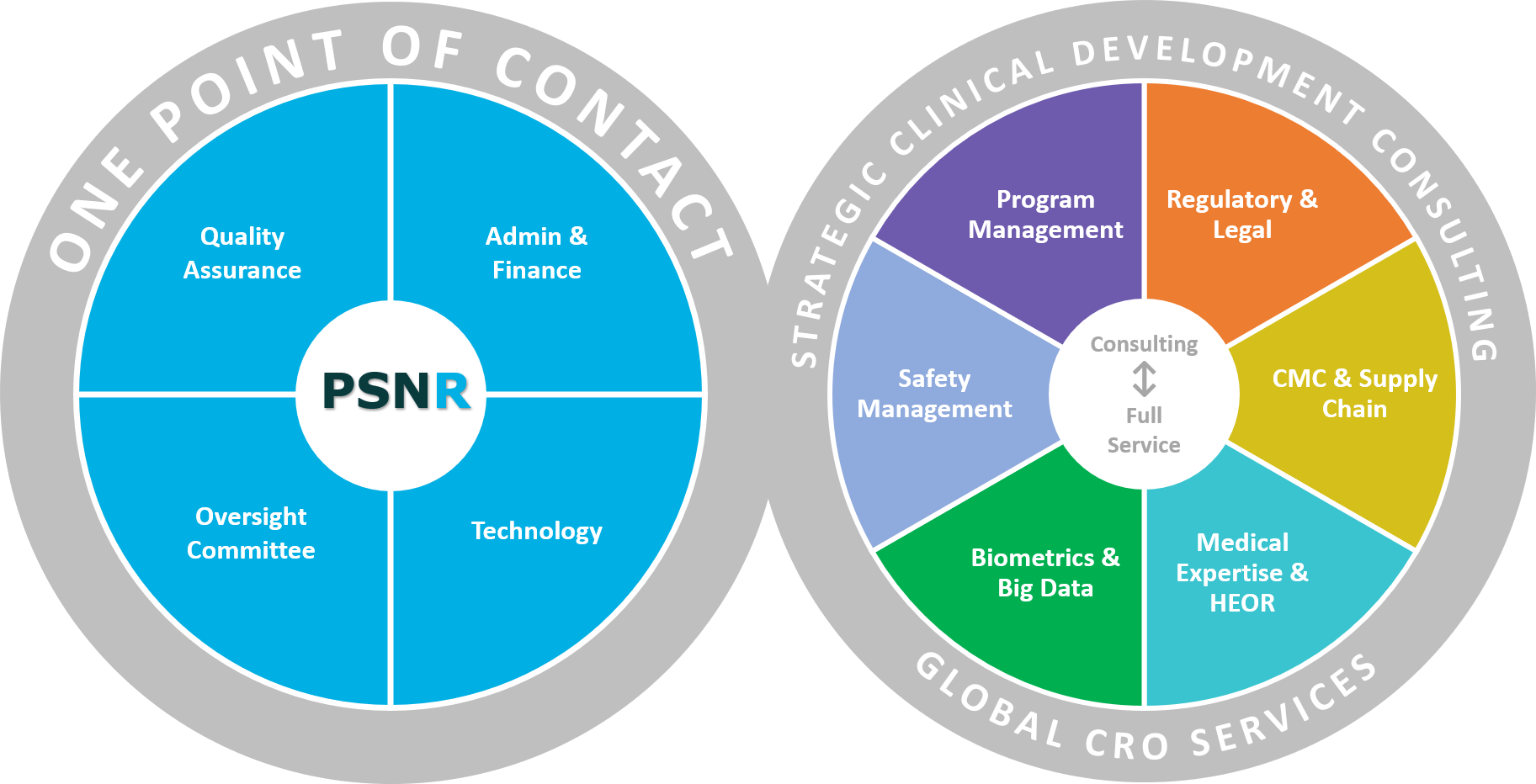
CMC Development Strategies for Small Pharma | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services
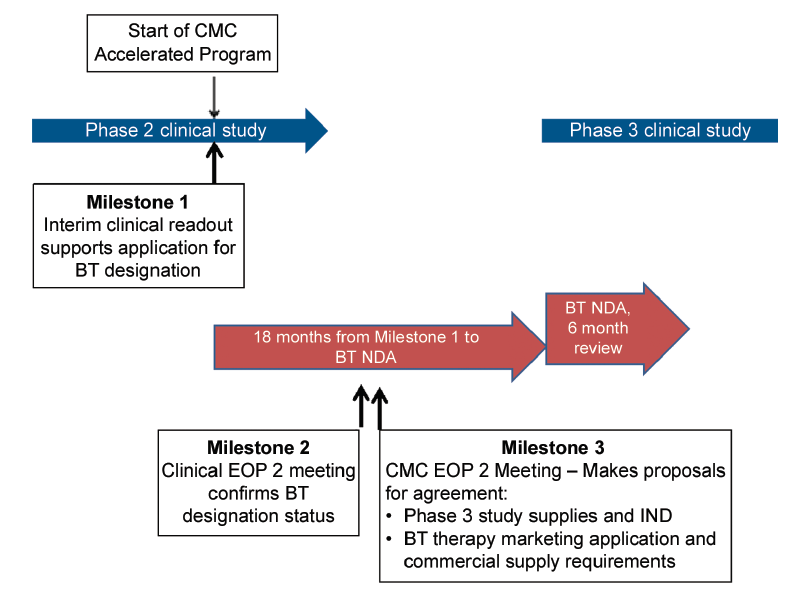
CMC Considerations when a Drug Development Project is Assigned Breakthrough Therapy Status | Pharmaceutical Engineering

CMC Development Strategies for Small Pharma | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

PET probe IND application components. Chemistry, Manufacturing, and... | Download Scientific Diagram

Phase-Appropriate CMC Activities Facilitate the Transition from Early Development through Commercialization