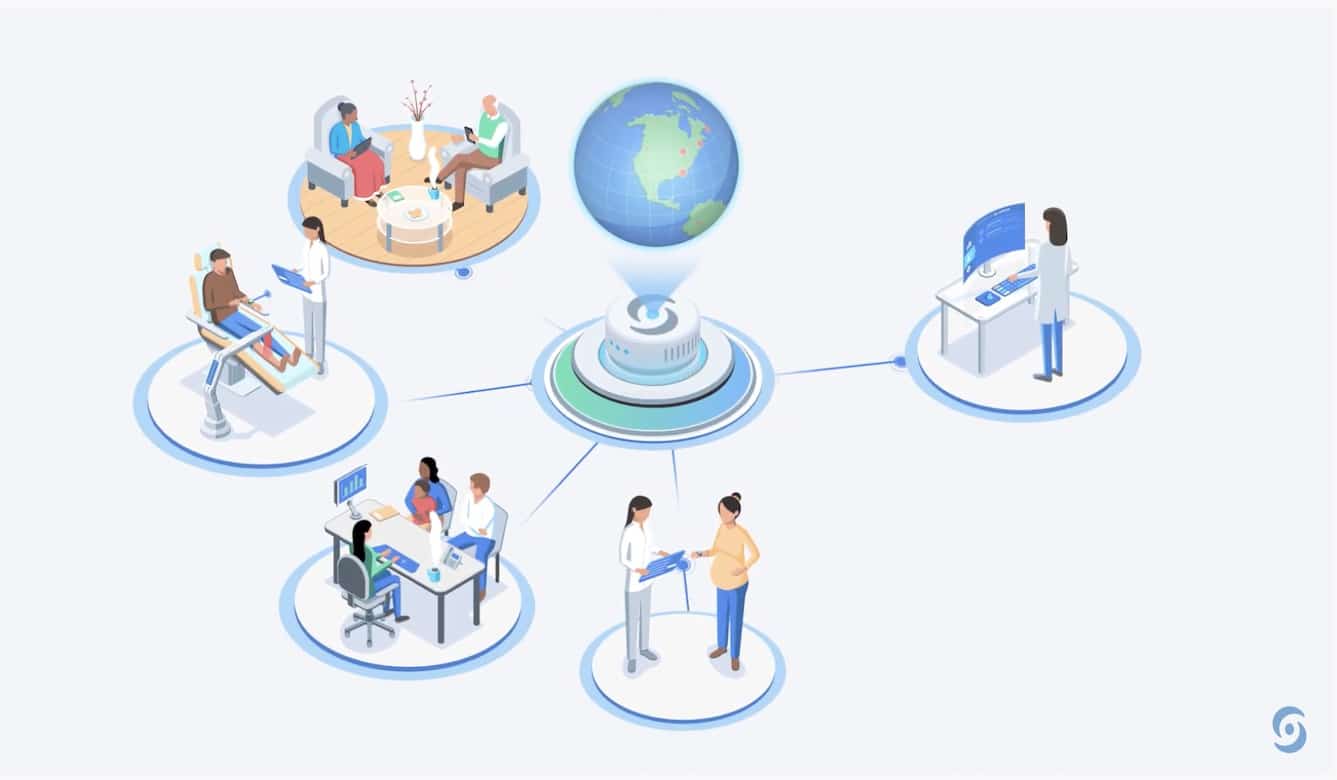
Overview of patient enrollment, clinical follow-up and personalized... | Download Scientific Diagram

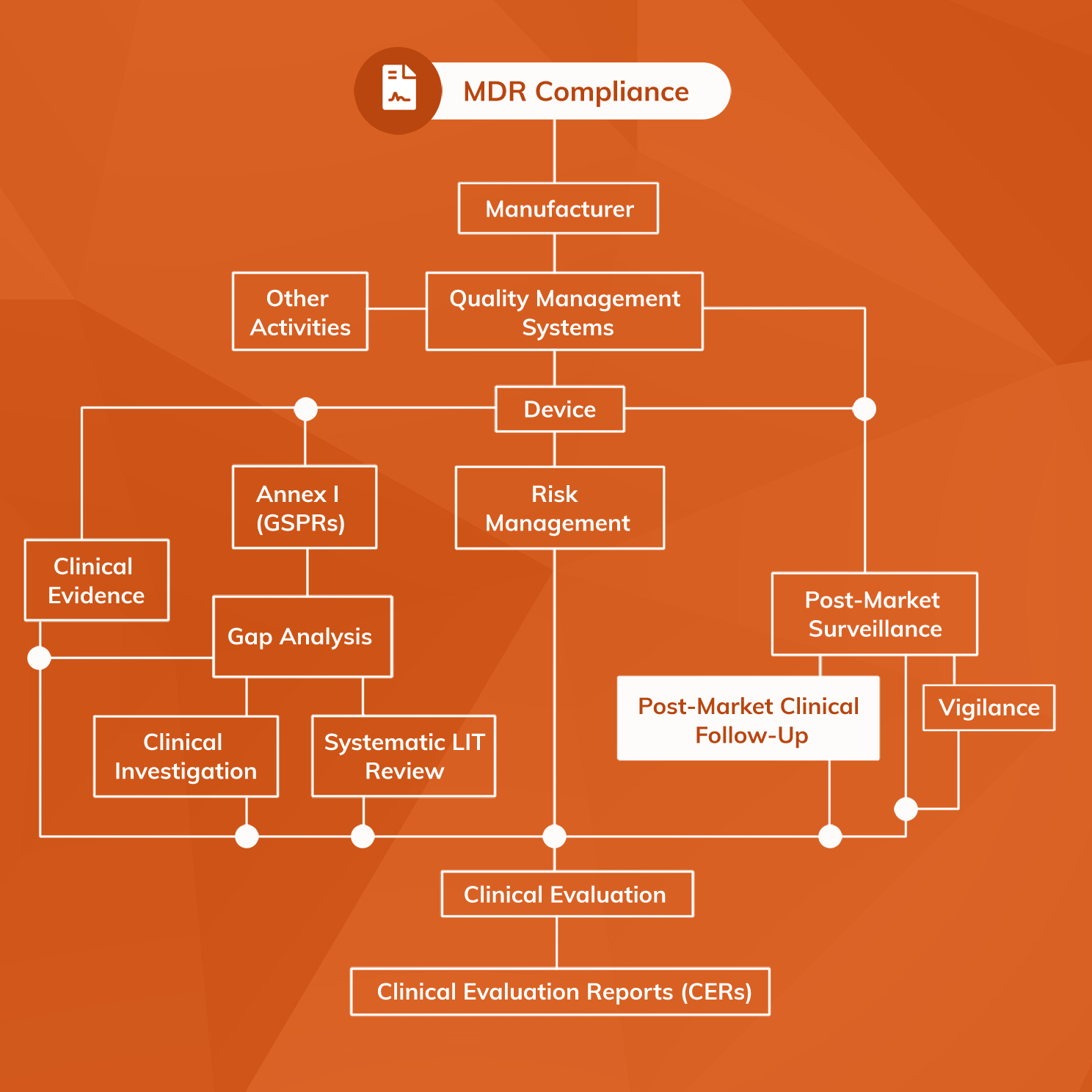
Does Your Organization's Post-Market Clinical Follow-Up (PMCF) Plan Adequately Reflect the Intensity Required in the Clinical Evaluation Report (CER) Under the Newest Medical Device Regulations? - Criterion Edge





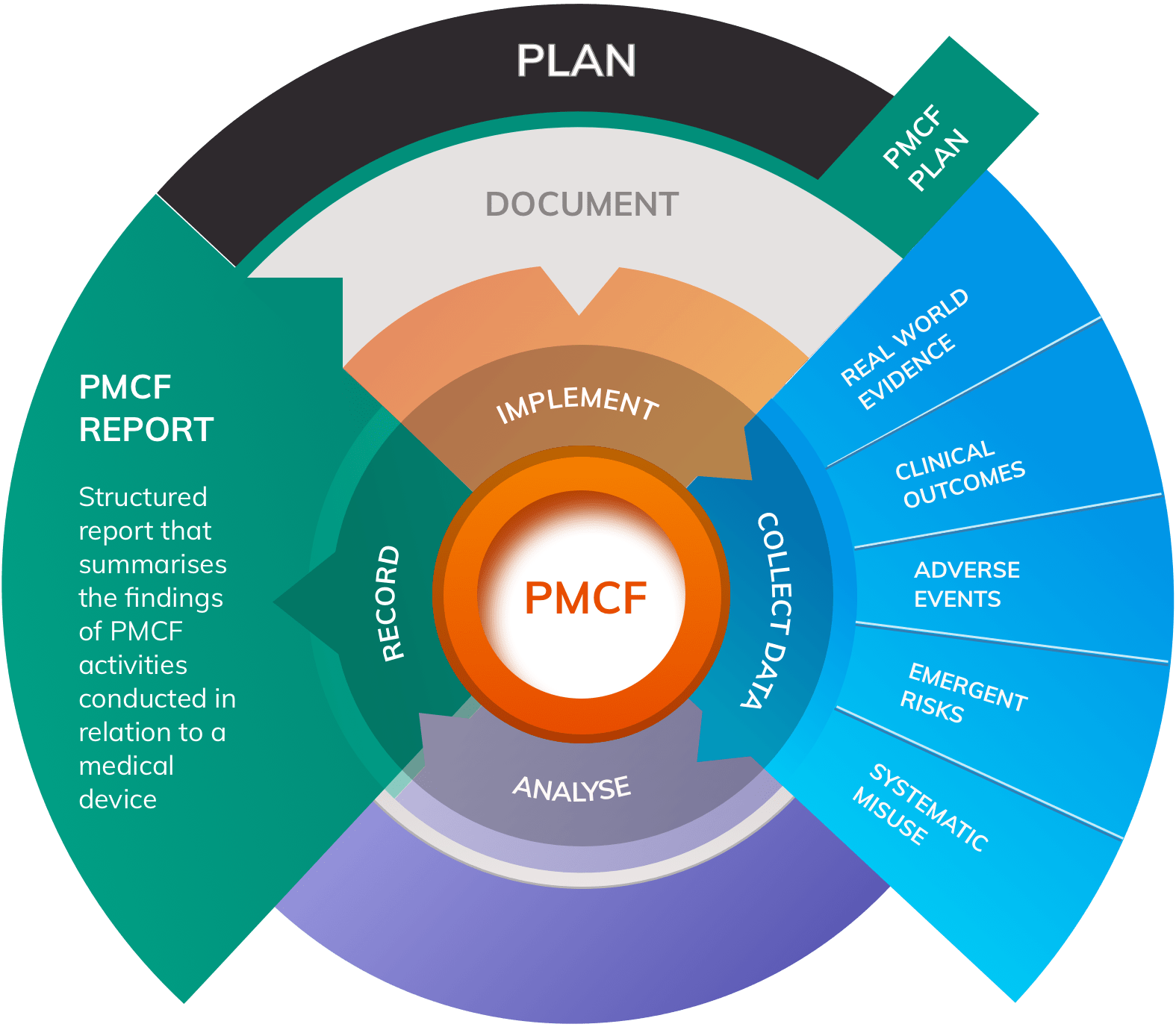





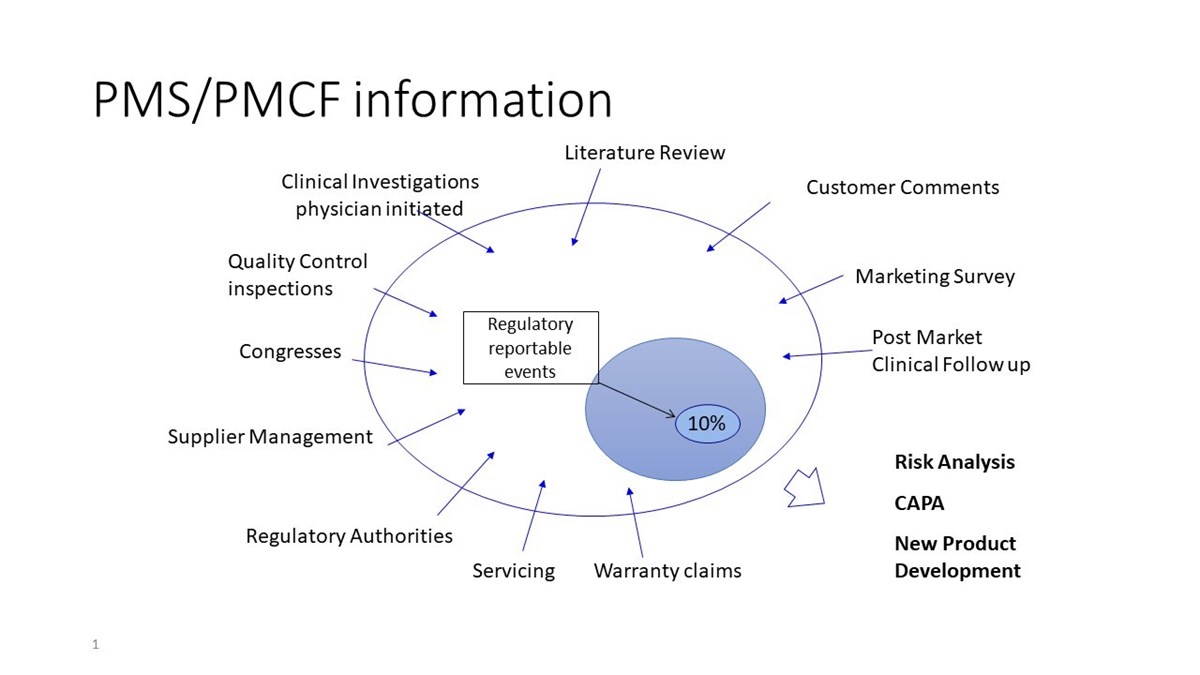



![Post-Market Clinical Follow-up Plan [ISO 13485 templates] Post-Market Clinical Follow-up Plan [ISO 13485 templates]](https://advisera.com/wp-content/uploads//sites/14/2021/08/18.4_Appendix_4_Post_Market_Clinical_Follow_up_Plan_Integrated_Preview_EN.png)