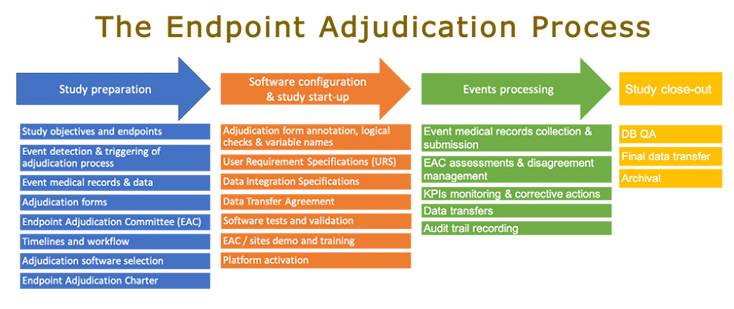
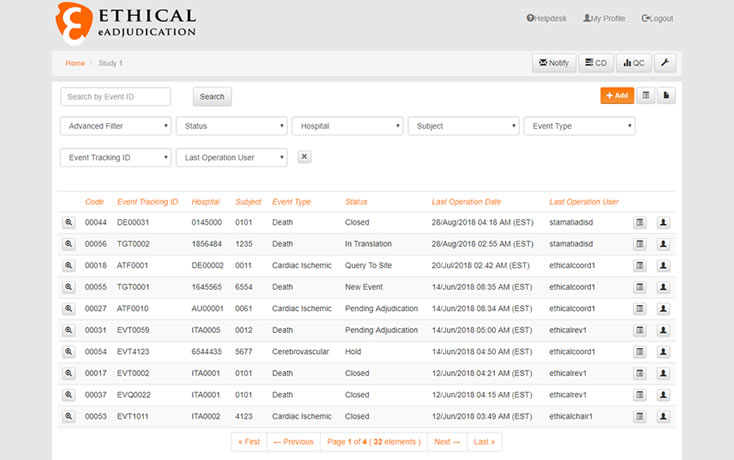
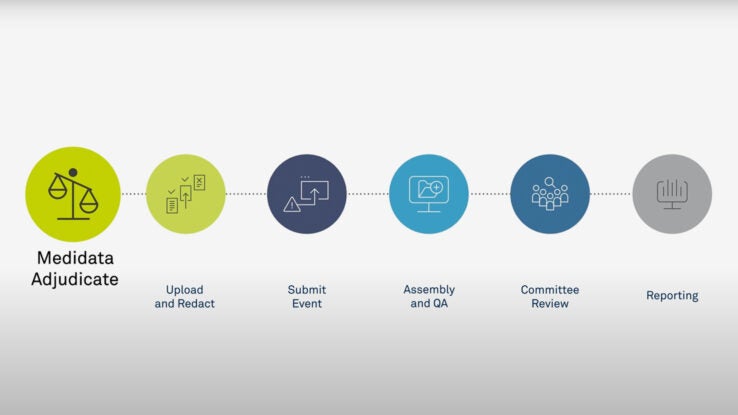
7 Areas to Evaluate When Selecting Your Clinical Adjudication Partner - Paperpicks Leading Content Syndication and Distribution Platform

Cardiovascular adverse events in the drug‐development program of bupropion for smoking cessation: A systematic retrospective adjudication effort - Kittle - 2017 - Clinical Cardiology - Wiley Online Library

Clinical endpoint adjudication in a contemporary all-comers coronary stent investigation: Methodology and external validation - ScienceDirect

Methodology of a reevaluation of cardiovascular outcomes in the RECORD trial: Study design and conduct - ScienceDirect
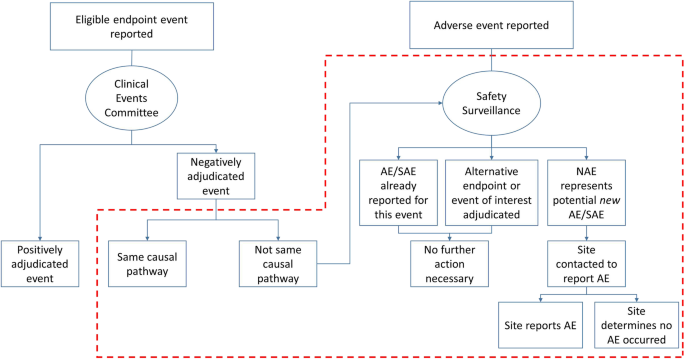
Methods for safety and endpoint ascertainment: identification of adverse events through scrutiny of negatively adjudicated events | Trials | Full Text
GLASSY design. CEC, Clinical Event Committee; GLASSY, GLOBAL LEADERS... | Download Scientific Diagram

Clinical events classification (CEC) in clinical trials: Report on the current landscape and future directions — proceedings from the CEC Summit 2018 - ScienceDirect

Determination of Hospitalization Type by Investigator Case Report Form or Adjudication Committee in a Large Heart Failure Clinical Trial (BEST) - Journal of Cardiac Failure
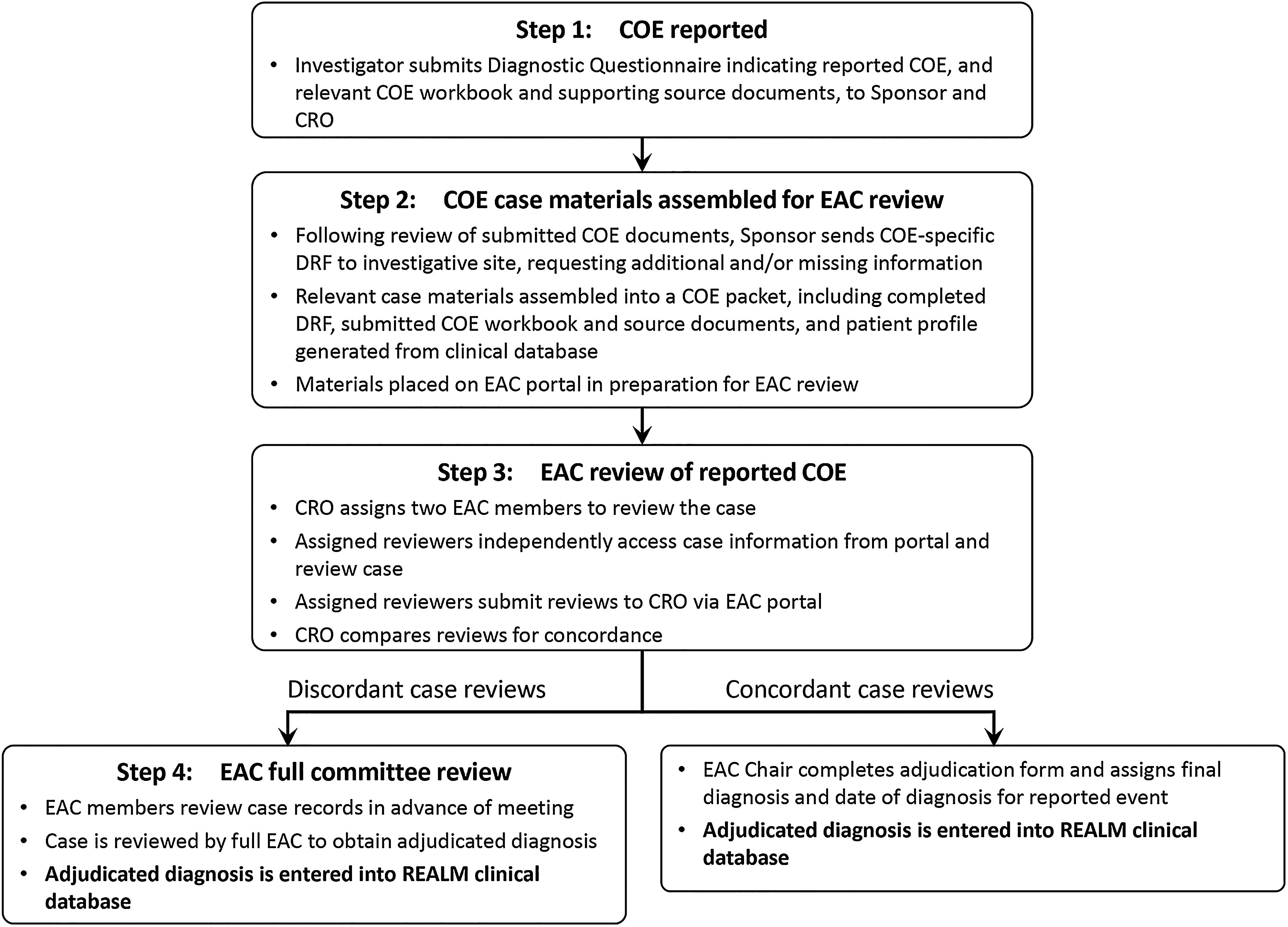
Clinical Outcome Event Adjudication in a 10-Year Prospective Study of Nucleos(t)ide Analogue Therapy for Chronic Hepatitis B

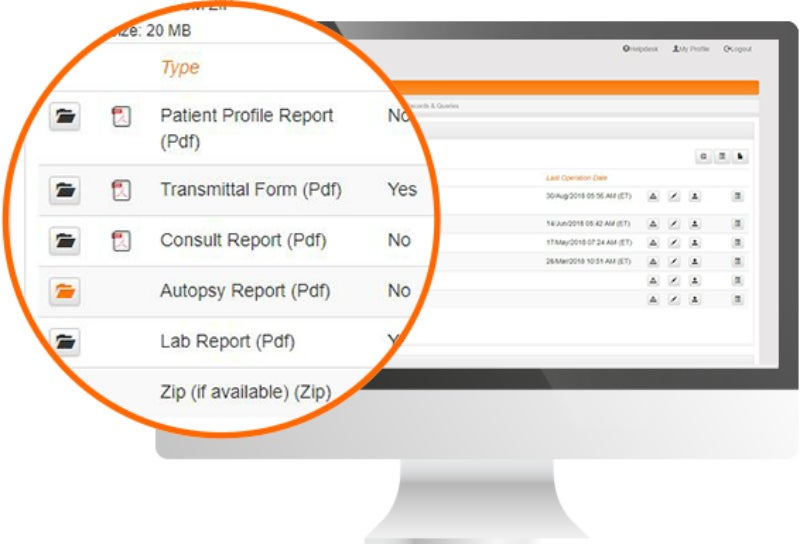
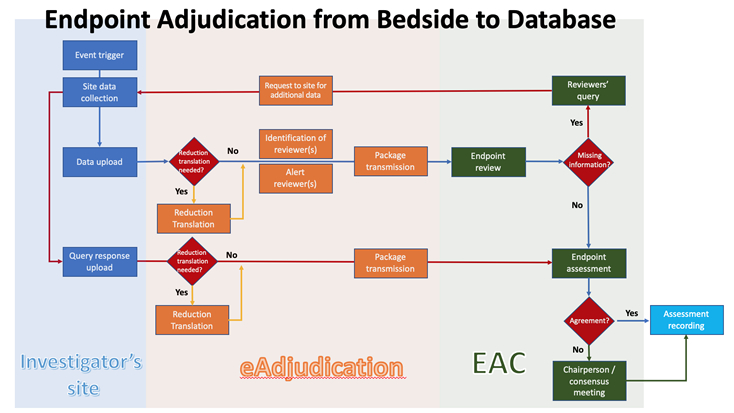
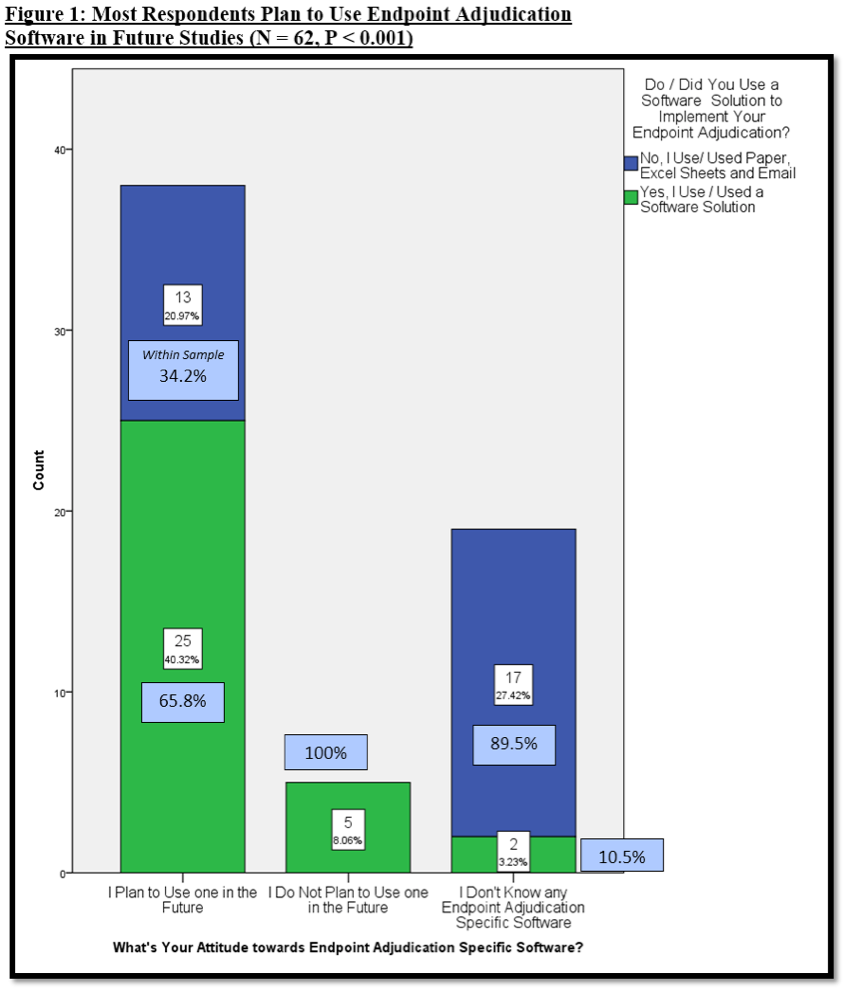
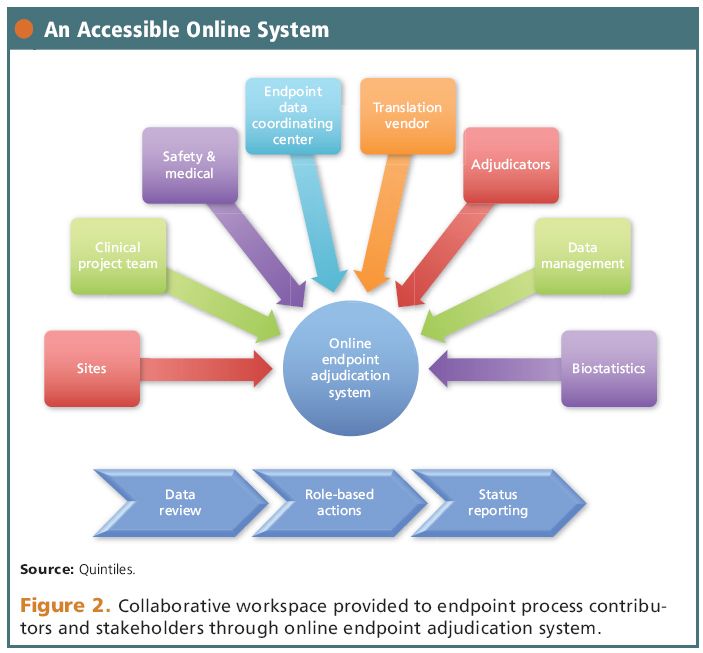
Biopharmaceutical and medical device companies have made EDC (electronic data capture) a cornerstone of clinical trial and registry management because of its proven, significant process improvements over paper-based systems. EDC is a technology ...
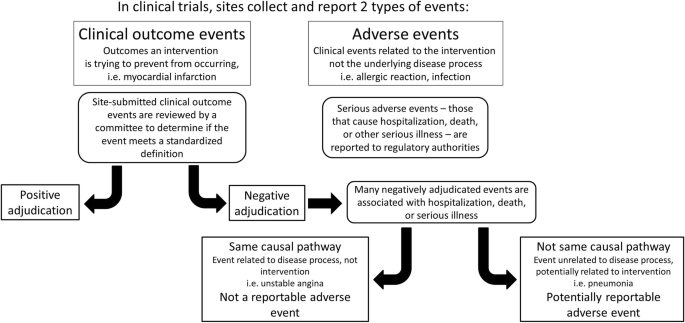
Methods for safety and endpoint ascertainment: identification of adverse events through scrutiny of negatively adjudicated events | Trials | Full Text

Comparative Reductions in Investigator-Reported and Adjudicated Ischemic Events in REDUCE-IT - ScienceDirect

Agreement between public register and adjudication committee outcome in a cardiovascular randomized clinical trial Erik Kjøller, MD, DMSc, Jørgen Hilden, - ppt download